
 EXECUTIVE SUMMARY
EXECUTIVE SUMMARY

Through the innovative power of bimiralisib, Torqur is aiming to redefine skin cancer prevention and treatment by addressing the unmet needs of patients with actinic keratosis (AK).
By targeting the PI3K/mTOR pathway , driver of the AK, our proprietary technology achieves promising clinical outcome, with favorable tolerability. As compared to 177-Lutetium-PSMA-617, the only commercial prostate cancer RLT
up to
100%
Response
in
animal
models
Cmiljanovic et al. “Topical PI3K/mTOR inhibitor PQR309
(Bimiralisib) in Actinic Keratoses and Cutaneous Squamous Cell
Carcinoma”, submitted
58m
US Patient
Population
https://www.skincancer.org/skin-cancer-information/actinic-keratosis,
https://www.coherentmarketinsights.com/market-insight/actinic-keratosis-market-1006
$9b
Addressable
Market
Ratushny et al. 2012 "From keratinocyte to cancer: the
pathogenesis and modeling of cutaneous squamous cell carcinoma."
J Clin Invest 122(2): 464-472
 THE PROBLEM
THE PROBLEM
1st
Most Common
Precancer
https://www.skincancer.org/skin-cancer-information/actinic-keratosis
60%
Squamous cell
carcinoma
diagnoses
are
caused by AK
https://www.skincancer.org/skin-cancer-information/squamous-cell-carcinoma/scc-causes-and-risk-factors
58m
Cases
In the U.S.
https://www.coherentmarketinsights.com/market-insight/actinic-keratosis-market-1006
85%
Of patients are
unaware of AK’s
existence
Almirall 2023 “85% of people unaware of actinic keratosis,
the most common pre-cancerous skin condition” Press
Release

Actinic Keratosis (AK) is the most common precancerous skin condition caused by chronic sun exposure. Filosa and Filosa 2015 "Actinic keratosis and squamous cell carcinoma: clinical and pathological features." G Ital Dermatol Venereol 150(4): 379-384 Huang et al. 2019 "Updates on Treatment Approaches for Cutaneous Field Cancerization." Curr Dermatol Rep 8(3): 122-132e
Despite being the number one cause of squamous cell carcinoma (SCC), AK remains notoriously underdiagnosed. Morton et al. 2023 "Expert Recommendations on Facilitating Personalized Approaches to Long-term Management of Actinic Keratosis: The Personalizing Actinic Keratosis Treatment (PAKT) Project." Acta Derm Venereol 103: adv6229
 ACTINIC KERATOSIS(AK)
ACTINIC KERATOSIS(AK)
WHAT IS ACTINIC KERATOSIS (AK)?
- The most common cutaneous condition treated by dermatologists.
- Dry pigmented papules or patches mainly in chronically sun-exposed skin areas.
PREVALENCE:
- In the U.S, over 35 million cases were treated for AK in 2015, accounting for more than 14% of all dermatology visits and with healthcare expenditure of approximately $3.1 billion.
- Prevalence rates of 40-60% in adults in Australia and of 5-31% in adults in Europe.
TREATMENT REQUIRED DUE TO:
- Risk for a malignant transformation (ca. 16% of AK progress to invasive cutaneous squamous cell carcinoma (iSCC).
- Immuno-compromised patients are at significantly increased risk to develop non-melanoma skin cancer (NMSC).
- Cosmetic problems, as > 80% appear on visible parts of the body such as the head, neck, dorsal surface of the hands and forearms, and they may negatively impact the quality of life when lesions become unsightly.
RISK FACTORS:
- >45 years old males
- Light hair and eye color
- Sunburns in childhood and adulthood
- Chronic occupational and/or recreational sun exposure
13m AK treatments
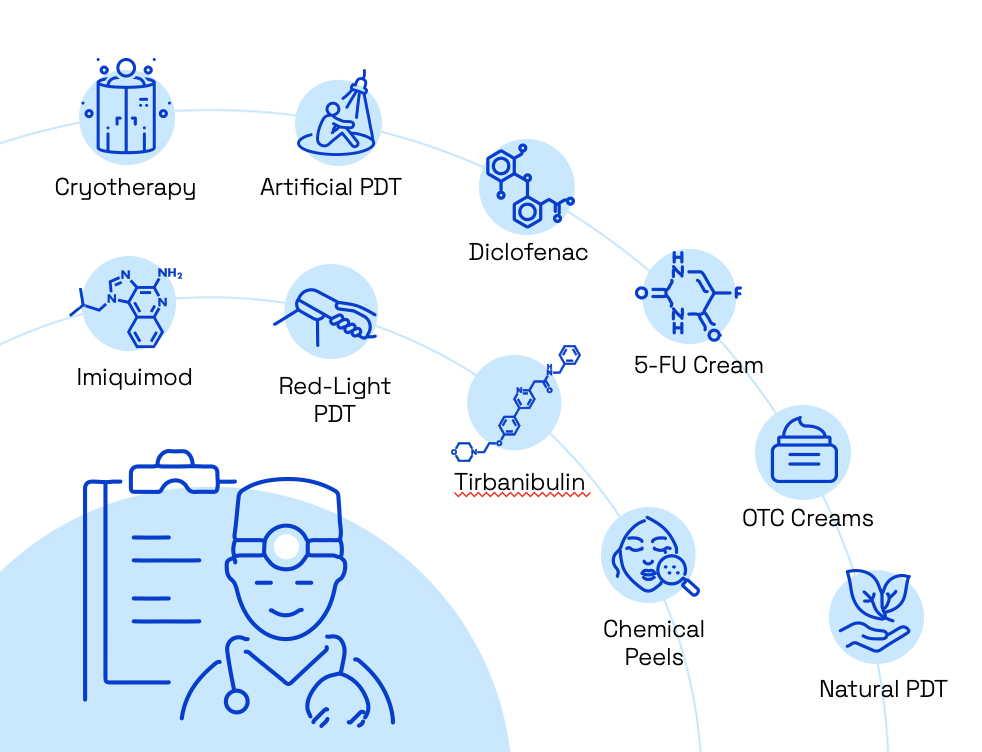
Over 13 million AK treatments are performed each year in the U.S., yet no standard of care exists for this chronic condition. Hedberg et al. 2022 "Molecular Mechanisms of Cutaneous Squamous Cell Carcinoma." Int J Mol Sci 23(7) Stockfleth et al. 2016, Stockfleth 2017, Hashim et al. 2019, Huang et al. 2019, Del Regno et al. 2022
up to ~58% recurrence rates
All of the available care avenues suffer from efficacy, tolerability or access shortcomings, with exceptionally high recurrence rates. Morton et al. 2023. "Expert Recommendations on Facilitating Personalized Approaches to Long-term Management of Actinic Keratosis: The Personalizing Actinic Keratosis Treatment (PAKT) Project." Acta Derm Venereol 103: adv6229
Fragmented landscape with
10+
treatment options
All current treatment options fail to deliver lasting results, with
high recurrence rates even for the most effective short-term
solutions.
Hedberg et al. 2022 "Molecular Mechanisms of Cutaneous Squamous
Cell Carcinoma." Int J Mol Sci 23(7) Stockfleth et al. 2016,
Stockfleth 2017, Hashim et al. 2019, Huang et al. 2019, Del Regno
et al. 2022
Morton et al. 2023. "Expert Recommendations on Facilitating
Personalized Approaches to Long-term Management of Actinic
Keratosis: The Personalizing Actinic Keratosis Treatment (PAKT)
Project." Acta Derm Venereol 103: adv6229

1st
Most Common
Cancer in Men
42,000
Cases In the U.S.
1 in 8
Men will be
diagnosed during
their life
<30%
Survival rate of
stage IV patients

Prostate cancer is the most common cancer amongst men and the 2nd leading cause of cancer-related deaths in the U.S.
The disease becomes particularly deadly once it reaches its later stages. Metastatic prostate cancer is typically resistant to surgical treatment and carries only a <30% survival rate over 5 years.
1st
Most Common
Cancer in Men
42,000
Cases In the U.S.
1 in 8
Men will be
diagnosed during
their life
<30%
Survival rate of
stage IV patients
12,230
Deaths in the
U.S. each year
60%
Disease 5-year
survival rate
Top 5
Most prevalent
Cancer globally
434,915
Patients living in the U.S. with Head & Neck cancer

The prevalence of Head and Neck Cancers (cancers of the nasal cavity, sinuses, lips, mouth, larynx or pharynx) has been on a continuous rise globally. One of the risk factors is the HPV virus, carried by more than 42 million Americans.
Head and neck cancers are considered among the most difficult to treat due to their close proximity to key organs and glands.
Torqur AG is a Swiss biotech innovator redefining precancer care with bimiralisib, a transformative therapy targeting actinic keratosis (AK). Torqur AG aims to deliver cutting-edge, patient-focused solutions.

Topical bimiralisib has proven to be well tolerated and to be effective in both SCC and AK mouse models.

In a genetically modified mouse model daily topical bimiralisib induced complete regression of SCC in 4 weeks post treatment, with excellent tolerability and lack of common side effects such as ulceration.

In an AK mouse model (immuncompetent hairless UV-B-irradiated mice) topical daily bimiralisib resulted in substantially inhibition of the pathological evolution minimizing the risk of AK progression.
Figures reformatted from: Cmiljanovic et al. “Topical PI3K/mTOR inhibitor PQR309 (Bimiralisib) in Actinic Keratoses and Cutaneous Squamous Cell Carcinoma”, submitted
A randomized phase 2 proof-of-concept study to evaluate the efficacy and safety of topical bimiralisib application in patients suffering from actinic keratosis on the face and/or scalp and/or back of hands over a 2- and 4-week treatment period.
Neck and head cancers, particularly squamous cell carcinomas, present unique treatment challenges due to their proximity to vital organs and high recurrence rates. Innovative solutions are essential to improve outcomes.
Prostate cancer, particularly advanced cases, is often driven by PTEN-LoF mutations. Innovative therapies are transforming its treatment.
Download Our
Company Presentation
your name
your email
organization name
